STATISTICAL MECHANICS
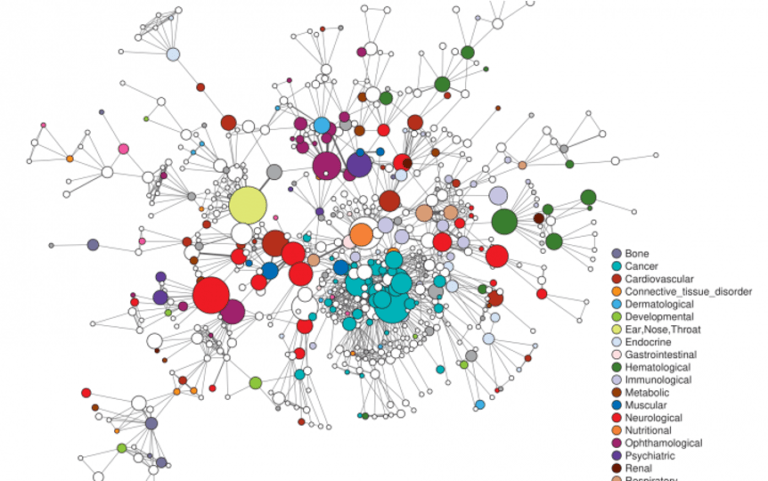 DISEASOME. Networks can represent the relationship between diseases and the malfunctioning of genes and/or microbiota. This is the first step of Personalised Medicine
DISEASOME. Networks can represent the relationship between diseases and the malfunctioning of genes and/or microbiota. This is the first step of Personalised Medicine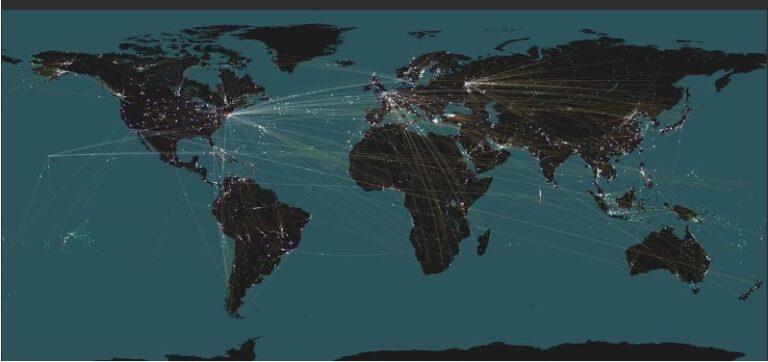
EPIDEMICS. Networks are the structure on which viruses and their carriers (i.e. people) travel. The study of societal interconnections has proved essential in the management of COVID-19
Soft Matter (Achille Giacometti, Flavio Romano, Tatjana Škrbić)
Self-Assembly of semi flexible polymers in solution
Formation of a membrane of DPCC in water
Folding of a small proteins in water
Self-hybridization of two single-strands of DNA
Solvent quality versus solvent polarities

We report on extensive molecular dynamics atomistic simulations of a meta-substituted poly-phenylacetylene (pPA) foldamer dispersed in three solvents, water H2O, cyclohexane cC6H12, and n-hexane nC6H14, and for three oligomer lengths 12mer, 16mer and 20mer. At room temperature, we find a tendency of the pPA foldamer to collapse into a helical structure in all three solvents but with rather different stability character, stable in water, marginally stable in n-hexane, unstable in cyclohexane. In the case of water, the initial and final number of hydrogen bonds of the foldamer with water molecules is found to be unchanged, with no formation of intrachain hydrogen bonding, thus indicating that hydrogen bonding plays no role in the folding process. In all three solvents, the folding is found to be mainly driven by electrostatics, nearly identical in the three cases, and largely dominant compared to van der Waals interactions that are different in the three cases.
This scenario is also supported by the analysis of distribution of the bond and dihedral angles and by a direct calculation of the solvation and transfer free energies via thermodynamic integration. The different stability in the case of cyclohexane and n-hexane notwithstanding their rather similar chemical composition can be traced back to the different entropy-enthalpy compensation that is found similar for water and n-hexane, and very different for cyclohexane.
A comparison with the same properties for poly-phenylalanine oligomers underscores the crucial differences between pPA and peptides.
To highlight how these findings can hardly be interpreted in terms of a simple “good” and “poor” solvent picture, a molecular dynamics study of a bead-spring polymer chain in a Lennard-Jones fluid is also included.
Work by Cedrix Dongmo Post-doc in our group.
C.J. Dongmo, T. Arcangeli, T. Skrbic, and A. Giacometti
Solvent Quality and Nonbiological Oligomer Folding: Revisiting Conventional Paradigms
Soft Matter 20, 6507-6527 (2024)
doi:10.1039/d4sm00727a
[arxiv][pdf]
Self-Assembly of Janus helices

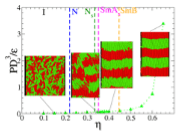
The phase diagram of hard helices differs from its hard rods counterpart by the presence of chiral “screw” phases stemming from the characteristic helical shape, in addition to the conventional liquid crystal phases also found for rod-like particles. Using extensive Monte Carlo and Molecular Dynamics simulations, we study the effect of the addition of a short-range attractive tail representing solvent-induced interactions to a fraction of the sites forming the hard helices, ranging from a single-site attraction to fully attractive helices for a specific helical shape. Different temperature regimes exist for different fractions of the attractive sites, as assessed in terms of the relative Boyle temperatures, that are found to be rather insensitive to the specific shape of the helical particle. The temperature range probed by the present study is well above the corresponding Boyle temperatures, with the phase behaviour still mainly entropically dominated and with the existence and location of the various liquid crystal phases only marginally affected. The pressure in the equation of state is found to decrease upon increasing the fraction of attractive beads and/or on lowering the temperature at fixed volume fraction, as expected on physical grounds.
All screw phases are found to be stable within the considered range of temperatures with the smectic phase becoming more stable on lowering the temperature. By contrast, the location of the transition lines do not display a simple dependence on the fraction of attractive beads in the considered range of temperatures.
This was part of the PhD thesis of Laura dal Compare
L.Dal Compare, F. Romano, J.A.Wood, A. Widmer-Cooper, and A. Giacometti
Janus helices: From fully attractive to hard helices
J. Chem. Phys.159174905-1 174905-16 (2023)
Self-Assembly of semiflexible polymers in solution

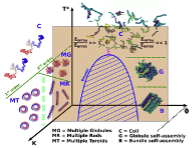
Using Langevin dynamics complemented by Wang-Landau Monte Carlo simulations, we study the phase behavior of single and multiple semiflexible polymer chains in solution under poor-solvent conditions. In the case of a single chain, we obtain the full phase diagram in the temperature-bending rigidity (stiffness) plane and we provide connections with a classical mean field result on a lattice as well as with past results on the same model. At low bending rigidity and upon cooling, we find a second order coil-globule transition, followed by a subsequent first order globule-crystal transition at lower temperatures. The obtained crystals have the shape of a twisted rod whose length increases with the increase of the stiffness of the chain. Above a critical value of the stiffness, we also find a direct first order globule-crystal transition, with the crystal having the form of a twisted toroid. Close to the triple point, we find a region with isoenergetic structures with frequent switching from rods to toroids, with the toroid eventually becoming the only observed stable phase at higher stiffness. The model is then extended to many thermally equilibrated chains in a box and the analogous phase diagram is deduced where the chains are observed to first fold into a globule bundle at low stiffness upon cooling, and then rearrange into a nematic bundle via a nucleation process involving an isotropic-nematic transition. As in the single chain counterpart, above a critical stiffness the chains are observed to undergo a direct transition from a gas of isotropically distributed chains to a nematic bundle as the temperature decreases in agreement with recent suggestions from mean field theory. The consequences of these findings for self-assembly of biopolymers in solutions are discussed.
This was the Master thesis of Tobia Arcangeli.
T.Arcangeli, T. Skrbic, S. Azote, D. Marcato, A. Rosa, J.R. Banavar, R. Piazza, A. Maritan and A. Giacometti
Phase behaviour and self-assembly of semiflexible polymers in poor-solvent solutions
Macromolecules 57, 8940-8955 (2024)
doi: 10.1021/acs.macromol.4c01111
