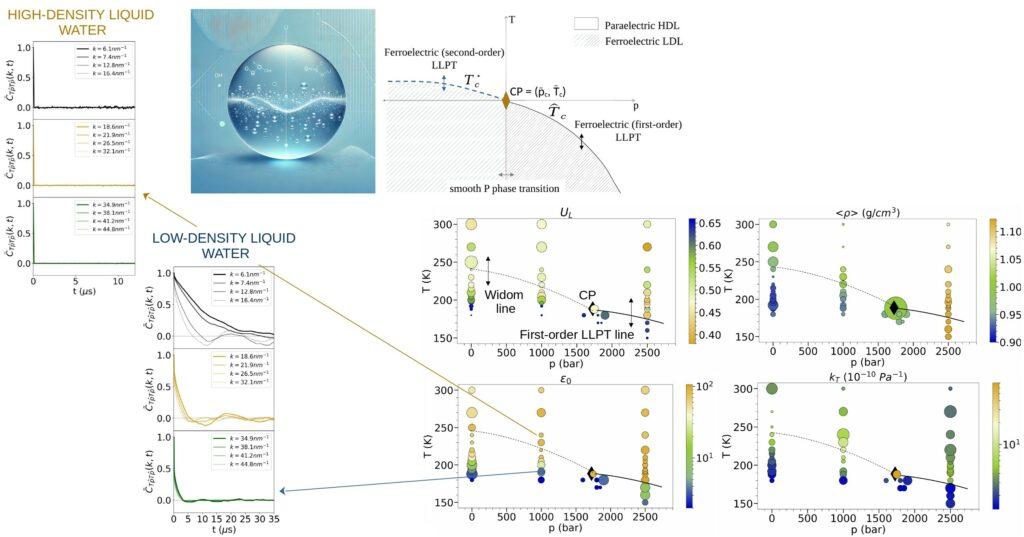
Water exhibits unique properties with respect to other liquids, so-called thermodynamic anomalies, even in its equilibrium state under Earth’s ambient conditions. Finding evidence nowadays in several molecular dynamics simulation studies, a compelling hypothesis for explaining the existence of water’s equilibrium anomalies is the so-called liquid-liquid phase transition (LLPT) between high-density and low-density liquid phases (HDL/LDL) in a supercooled, metastable liquid state. Its origin has been so-far not clarified. On the other hand, water is a polar liquid and, as such, can, in principle, undergo a ferroelectric phase transition, resulting in the setting of macroscopic polarization, under some thermodynamic conditions, even in the absence of an external electric field.
By analyzing extensive molecular dynamics simulations and developing a classical density functional theory in mean-field approximation, we unveil not only a link between ferroelectric and liquid-liquid phase transitions, but also the role of ferroelectricity in promoting the LLPT. Our theory treats water as a polar liquid. Grounded in the characteristics of the microscopic dipolar potential interaction and the liquid’s molecules positional disorder, it leads to a free energy expression that supports phase transitions both ferroelectric and liquid-liquid. The setting of macroscopic polarization occurs through a reorientation of molecular dipoles. This research not only characterizes but clarifies the origin of the LLPT and thermodynamic anomalies in water.
Understanding the origin of the peculiar behavior of water holds the key to uncovering fundamental mechanisms driving life and Earth’s processes. Our study explains the peculiarities of water while treating it as a generic polar liquid. Although the expression for free energy enabling phase transitions applies universally to polar liquids, its specific coefficients, which determine whether the transitions effectively occur under certain thermodynamic conditions, depend on the microscopic details of the liquid being studied. Our research thus raises significant questions: Could the ferroelectric properties of water, highlighted in this study, influence the natural selection of organisms and Earth’s geological evolution? Is water ‘merely’ a polar liquid with the microscopic characteristics suitable to allow a ferroelectric phase transition close to Earth’s environmental conditions? And what contributes to shaping its microscopic characteristics?
The link between liquid-liquid and ferroelectric phase transitions, established in this research, can, moreover, guide targeted experiments to measure the critical point of the liquid-liquid phase transition, one of the unresolved tasks in this field.
The methodology exploited in this research has two key strengths. First, it tackles a long-standing scientific issue, such as the LLPT in water, from a clear and groundbreaking perspective, focusing on dipolar degrees of freedom and conceiving a link between ferroelectric and liquid-liquid phase transitions. Second, it combines state-of-art molecular dynamics simulations with an elementary theory, with each being essential to achieve an understanding of the scientific issue. This approach requires and stimulates demonstrative-logical and creative thinking. Ultimately, these two aspects proved to be interconnected.
This research work originated independently. Its finalization benefited from the support and collaborative environment provided within the PRIN project “Deeping our understanding of the Liquid–Liquid transition in supercooled water” grant 2022JWAF7Y, involving Prof. Francesco Sciortino (Sapienza University), Prof. Achille Giacometti (Ca’ Foscari University) and Prof. Renato Torre (University of Florence and LENS).
Publication: M. G. Izzo, J. Russo, G. Pastore, Proc. Natl. Acad. Sci. U.S.A. 121 (47) e2412456121, https://www.pnas.org/doi/10.1073/pnas.2412456121 (2024)